

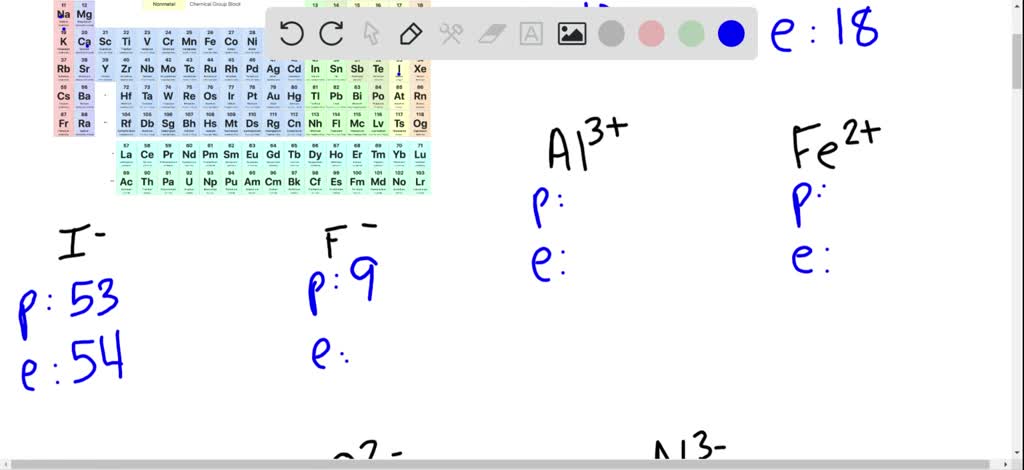
Since you're missing an electron, you aren't really a complete sodium atom either. That's the same number of electrons as neon (Ne). You have one less electron than your atomic number. You are still the element sodium, but you are now a sodium ion (Na +). That missing electron gives you a positive charge. You are also an ion and missing one electron.
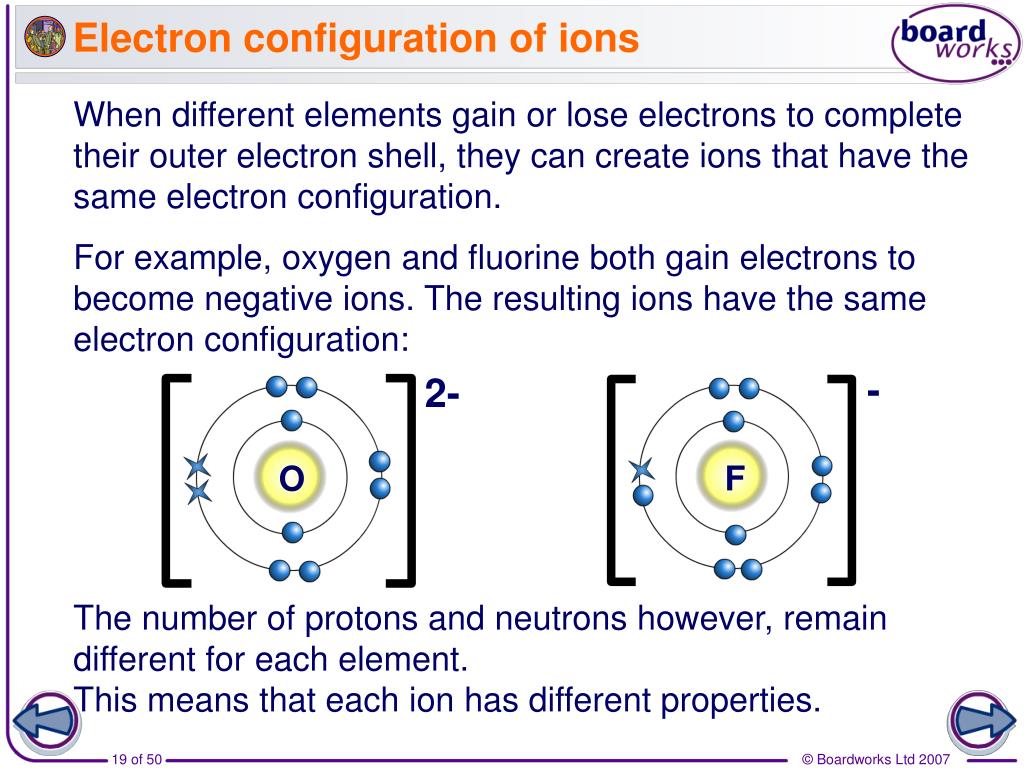
Chlorine has seventeen electrons and only needs one more to fill its third shell and be "happy." Chlorine will take your extra sodium electron and leave you with 10 electrons inside of two filled shells.
Negative ions have protons than electrons full#
Whenever an atom has full shells, we say it is "happy." Let's look at chlorine (Cl). When you lose that electron, you will you’ll have full shells. You need to find another element that will take that electron away from you. What do you do if you are a sodium (Na) atom? You have eleven electrons - one too many to have an entire shell filled. When you have an extra electron or two, you have a negative charge. When you are missing an electron or two, you have a positive charge. Ions are atoms with extra electrons or missing electrons. That means an atom with a neutral charge is one where the number of electrons is equal to the atomic number. A normal atom has a neutral charge with equal numbers of positive and negative particles. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.


 0 kommentar(er)
0 kommentar(er)
